“Fearfully and Wonderfully Made: Exploring Low Dose Naltrexone and the Divine Design of the Human Body”
Biohacking Endorphins and Enkephalins, the Peptides of Joy, Bliss while optimizing the Immune System.
The United States has experienced a significant number of opioid-related overdose deaths, which have skyrocketed over the last four years. According to the CDC.GOV under Drug Overdose Deaths in the United States, the total number of opioid-related reported deaths in the United States in the previous four years was approximately 394,000 and rising. This figure highlights the devastating impact of the opioid crisis during this period. I am sure the actual number of deaths is much higher. To give this some perspective, the total number of American servicemember deaths across World War II, the Korean War, and the Vietnam War combined is approximately 549,865. This includes battle deaths and other service-related fatalities.
The human body is masterfully equipped with a sophisticated system to manage physical pain, emotional distress, and the tenses of life. This system, known as the endorphin system, releases natural “painkillers” and “stress-relieving” hormones. These endorphins bind to their specialized opioid receptors, creating a built-in mechanism for soothing discomfort and restoring balance. It’s a testament to the intricacy and intentionality of our design.
In this highly complex system, opioid receptors exist throughout the human body, particularly in pain perception, emotional regulation, and physiological homeostasis. These receptors exist in the central and peripheral nervous systems and some non-neuronal tissues.
Brain: The central nervous system (CNS) contains the highest concentration of opioid receptors, pivotal in regulating pain perception, emotional responses, and autonomic functions. Within the brain, these receptors are concentrated in the midbrain, particularly in areas like the periaqueductal gray (PAG). This region serves as a central hub for pain modulation, processing sensory input, and pain signals while influencing the emotional aspects of pain and stress. This distribution helps explain why individuals can have varying pain thresholds, as their opioid receptor activity and response may differ.
The prefrontal cortex is another critical area where opioid receptors are abundant. Here, they integrate pain's cognitive and emotional aspects, allowing us to learn from painful experiences and adjust our behavior accordingly. For instance, if Bruce Lee were to hit you, the cognitive and emotional processing facilitated by your prefrontal cortex would likely teach you to avoid provoking him in the future—a clear example of the “survival mechanism” at work. This sophisticated system ensures physical protection and the development of adaptive behaviors that enhance survival.
Immune System: Our Immune Cells have Opioid receptors like macrophages, monocytes, and lymphocytes. These receptors can influence inflammation and immune responses.
Opioid receptors are also concentrated in the gastrointestinal (GI) system, which regulates motility and secretion. In clinical practice, especially among patients receiving high doses of synthetic opioids, we often encounter opioid-induced bowel dysfunction, a condition marked by severe constipation. During my time as an ICU intensivist, I frequently faced cases so severe that they required manual bowel dis-impaction.
God designed us with an intrinsic, powerful system for analgesia, pleasure, and bliss—the opioid system, a beautiful testament to the elegance of human biology. This system allows us to experience joy and manage pain by tapping into the natural production of endorphins and enkephalins.
One of the most tangible examples of this system in action is the phenomenon known as “runner’s high.” For athletes, particularly runners, a significant part of the joy in their sport comes from this euphoric sensation. It’s not just the act of running but the release of enormous amounts of endorphins and enkephalins that create a sense of pure bliss, emotional elevation, and even spiritual connection. It’s an awe-inspiring reminder of how the body is intricately wired—not just to endure but to thrive and experience joy.
The Three Amigos of Pain Relief: Types of Opioid Receptors
There are three main classes of opioid receptors, each with its unique personality and role in the body’s response to pain and emotion:
1. Mu (μ) Receptors: These are the heavy hitters of the opioid world, found in high brain and spinal cord concentrations. You thank them for pain relief (analgesia) and that warm, fuzzy feeling of euphoria.
2. Delta (δ) Receptors: These unsung heroes hang out in peripheral sensory neurons and immune cells. They specialize in mood regulation and lend a hand with pain relief, keeping things balanced in their low-key way. Think of them as the quiet friend in your group who always knows how to lift your spirits.
3. Kappa (κ) Receptors: The wildcard of the trio, kappa receptors are scattered throughout the brain, spinal cord, and peripheral tissues. They provide pain relief but add a twist with sedation and even a touch of dysphoria—like the prankster who gives you relief but also reminds you not to get too comfortable.
These three types form a finely tuned orchestra of pain modulation and emotional regulation.
Enkephalins are smaller peptides composed of just five amino acids; they primarily bind to delta-opioid receptors (DOR), with some activity at mu-opioid receptors (MOR). This specificity allows them to function in localized pain modulation and emotional regulation. They synergize with endorphins to manage pain, regulate stress, and fine-tune the immune system.
A key distinction lies in their regulation of neurotransmitters. Enkephalins are critical in modulating dopamine, serotonin, epinephrine, and norepinephrine, influencing mood, reward pathways, and stress responses. Endorphins, being larger molecules, have broader systemic effects, contributing to powerful pain relief (analgesia) and euphoria.
“God is Good.”
In addition to exercise, our bodies release a wealth of endorphins during moments of intimacy, when we indulge in chocolate, and when we experience relaxation or creative flow. As I sit in my chair composing this post, I find myself in a state of creativity and relaxation, reflecting on the day's challenges and transforming them into something meaningful. This process naturally triggers the release of endorphins, enhancing my sense of satisfaction and well-being.
If what you’re reading resonates with you or brings you positivity, you’re probably experiencing the same endorphin boost as I am. Thank you, J.J., for encouraging me to write.
“Biohacking”
Experts like Dr. William Seeds often criticize the term “biohacking,” emphasizing that we don’t “hack” biochemistry but instead work to understand and optimize it. Despite this, the term remains popular, particularly among followers of figures like Dave Asprey, who discusses manipulating and rating the body’s systems to improve health or performance.
Ironically, the concept of biohacking is not new. In a troubling twist, man-made synthetic opioid-like recreational drugs flooding the American public are, in essence, a dark form of biohacking aimed at exploiting the endorphin system. These substances hijack the brain’s natural reward pathways, providing a fleeting sense of euphoria but leaving devastation in their wake.
Enter Naltrexone, a medication FDA-approved in 1984 for treating opioid and alcohol addiction at doses of 50–300 mg. At this dosage, naltrexone binds permanently to opioid receptors, effectively blocking the “high” that people with an addiction would experience from using street drugs. While this approach has helped many individuals avoid relapse, it raises an ethical question: Are we merely trading one dependency (street drugs) for another (a pharmaceutical drug that generates profit for Big Pharma)?
This dilemma highlights a deeper issue within the healthcare system—addressing addiction at its root cause versus managing it symptomatically. While naltrexone at higher doses has proven effective in blocking opioid-induced euphoria, its application at low doses (LDN) has opened new doors in treating autoimmune diseases, chronic pain, and inflammation, offering a potential way to optimize the body’s natural systems rather than perpetuate dependency.
Mechanism of Action: Unlocking the Body’s Natural Healing Potential
When used at low doses (0.5 mg to 4.5 mg), Low Dose Naltrexone (LDN) binds temporarily to mu-opioid receptors (MOR), partially blocking them for a short duration of 3–4 hours. This blockade is significantly shorter than the 24-hour or longer receptor blockade observed with higher doses (50–300 mg) used for opioid and alcohol addiction.
This brief interruption in receptor activity triggers a compensatory response. The receptors send a signal to the brain, prompting an adaptive upregulation that increases the production of the body’s natural endorphins and enkephalins—powerful peptides responsible for pain relief, emotional well-being, and immune regulation.
As a result, this temporary blockade enhances the body’s ability to:
• Modulate Pain: Amplify natural analgesic effects.
• Reduce Inflammation: Downregulate pro-inflammatory pathways.
• Stabilize Mood: Improve emotional resilience and well-being.
• Boost Immune Responses: Optimize immune system functionality.
This finely tuned mechanism highlights how LDN works synergistically with the body’s natural systems to promote healing and balance. It offers a unique and practical approach to managing chronic pain, inflammation, and immune-related conditions.
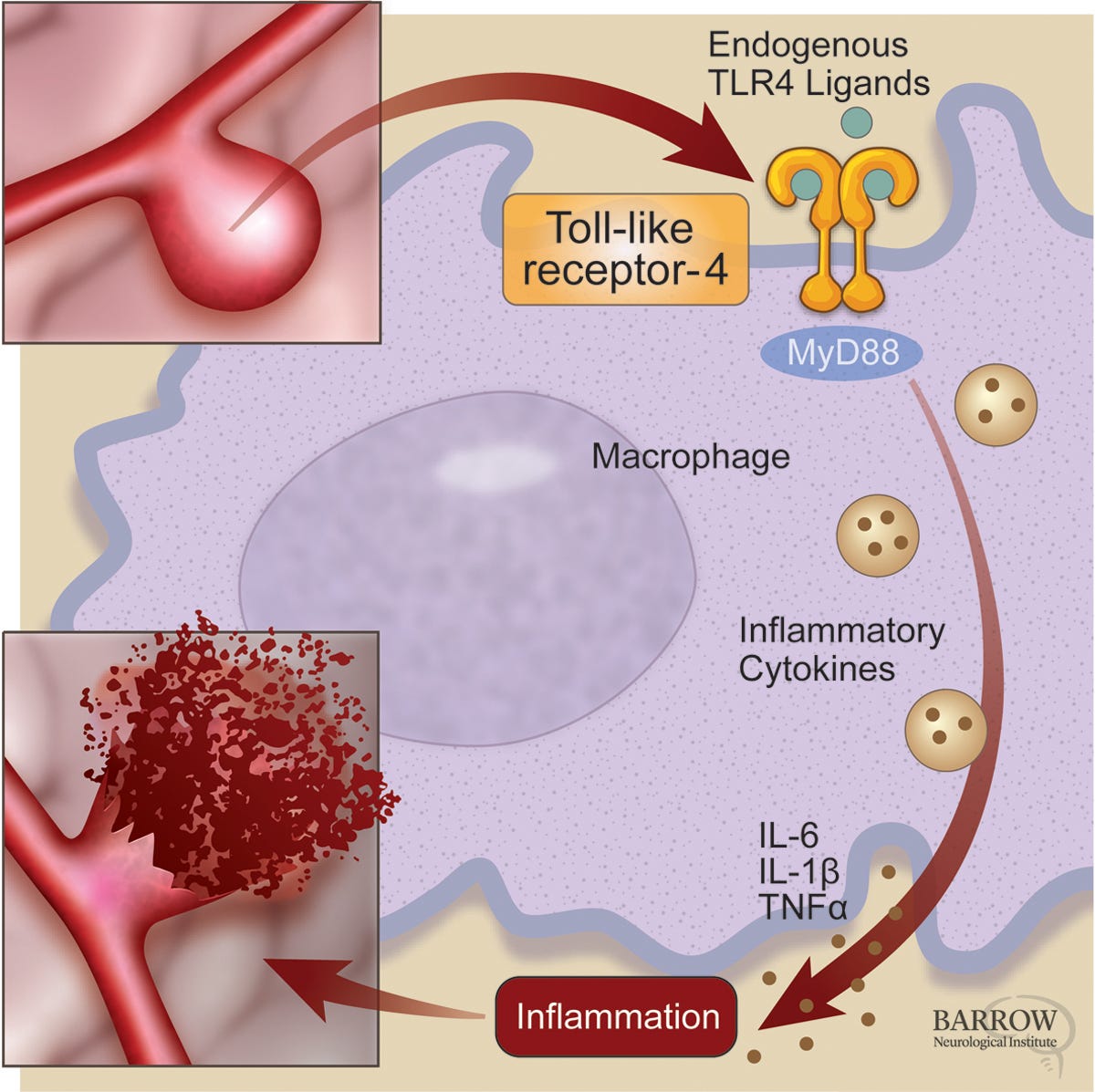
Toll-Like Receptor-4
Low Dose Naltrexone (LDN): A Dual-Action Modulator for Inflammation in a Toxic World
Low Dose Naltrexone (LDN) is a unique compound due to its isomeric nature, possessing both a left-handed (levo-naltrexone) and right-handed (dextro-naltrexone) configuration. This duality underpins its remarkable versatility in modulating the body’s natural systems:
1. Levo-Naltrexone: Temporarily blocks opioid receptors, triggering the body to upregulate the production of natural pain-relieving and mood-enhancing chemicals like endorphins and enkephalins.
2. Dextro-Naltrexone: Acts as an antagonist at Toll-like Receptor 4 (TLR4), a crucial component of the innate immune system.
In previous discussions, we explored how the immune system is divided into two branches:
• The Innate Immune System, our first line of defense, responds rapidly to threats.
• The Adaptive Immune System, which provides targeted and long-term protection.
Toll-like Receptor 4 (TLR4) is a key player in the innate immune response, found on immune cells such as macrophages, microglia, and dendritic cells. TLR4 is responsible for recognizing a variety of threats, including:
• Toxins
• Pathogens
• Damaged or senescent cells
When TLR4 is activated, it triggers a cascade of pro-inflammatory signals, releasing Cytokines like TNF-α, IL-1β, and IL-6 and Reactive Oxygen Species (ROS), which contribute to oxidative stress and tissue damage.
Living in 2025, our bodies are under relentless assault from environmental toxins and stressors: Heavy metals and pollutants in our air, fluoride, and other contaminants in our water supply, spike protein shedding from widespread exposure to jabbed individuals, chemical-laden food filled with preservatives, pesticides, and additives, and Electromagnetic Fields (EMFs). The proliferation of 5G and soon-to-be-6 G technologies creates “dirty energy,” further straining our systems.
As a result, nearly everyone lives in a state of chronic low-grade inflammation, which is increasingly recognized as the root cause of most diseases—from autoimmune disorders to cardiovascular diseases, neurodegeneration, and cancer. Much like Ivermectin, LDN boasts a remarkable anti-inflammatory profile with minimal side effects, making it a valuable tool in mitigating the impact of modern environmental stressors. It offers a natural, targeted approach to counteract the pervasive inflammatory state many people face today.
Unless you’re a Tibetan monk living in complete isolation from modern society, avoiding these toxic exposures is nearly impossible. However, LDN can bolster your body’s resilience and mitigate the effects of this designed poisonous environment.
LDN and Cancer Cell Growth
Low-dose naltrexone (LDN) exerts its therapeutic effects through the Opioid Growth Factor (OGF) and opioid Growth Factor Receptor (OGFr) pathways. These pathways control the cell cycle and regulate cell proliferation and tissue repair.
The OGF-OGFr Pathway: Opioid Growth Factor (OGF), also known as met-enkephalin, is a naturally occurring opioid peptide. Opioid Growth Factor Receptor (OGFr) is a specific receptor found on the surface of cells, particularly in rapidly dividing tissues.
OGF binds to OGFr, forming a complex that regulates cellular activities, particularly those related to growth and repair. This interaction tightly controls cell proliferation by modulating the cell cycle.
Harnessing Biochemistry for Cellular Health: Insights from William Seeds, MD
Attending a conference with William Seeds, MD, is a journey into the intricate world of cellular biochemistry. Dr. Seeds emphasizes the importance of memorizing biochemical pathways visually, as understanding and optimizing these pathways can profoundly influence cellular health.
Targeting specific pathways allows us to starve and isolate cancer cells, inducing them to undergo autophagy (programmed cellular death). It also allows us to reprogram senescent cells, guiding them toward autophagy or rescuing them to standard functionality.
One pathway Dr. Seeds highlights is the Opioid Growth Factor (OGF)—opioid Growth Factor Receptor (OGFr) pathway, a critical regulator of the cell cycle. This pathway works by modulating the expression of cell cycle inhibitors, helping maintain proper cellular growth and repair. A typical cell undergoes approximately 50 cell divisions during its lifespan (the Hayflick limit). The OGF-OGFr pathway controls cell cycle progression by upregulating two key inhibitors: P16 Cyclin-Dependent Kinase Inhibitor, which slows down the transition of the cell cycle from the G1 phase to the S phase.P21 Cyclin-Dependent Kinase Inhibitor: Reinforces the brakes, further ensuring controlled cell division. Uncontrolled cellular division is cancer.
Low-dose naltrexone (LDN) enhances the activity of these inhibitors and, through its influence on the OGF-OGFr pathway, slows excessive cell proliferation. This controlled regulation is critical for preventing cellular dysfunction. Optimizing the OGF-OGFr pathway has profound implications for conditions involving dysregulated cell growth:
Cancer: LDN slows the uncontrolled proliferation of tumor cells by enforcing proper cell cycle checkpoints and encouraging tumor cells to undergo autophagy, reducing their ability to grow and spread. In Autoimmune Diseases, it regulates the overactive immune response by modulating the proliferation of immune cells, restoring the balance to the immune system, reducing damage to healthy tissues, and promoting healing while preventing excessive scarring or fibrosis.
In the field of Longevity Science, many of my colleagues and professors advocate for and personally use Low Dose Naltrexone (LDN) as part of their anti-aging strategies. LDN provides a multifaceted approach to promoting longevity by addressing key pillars of healthy aging, including:
• Reducing chronic inflammation (‘inflammaging’), a major driver of age-related diseases.
• Optimizing the immune system by balancing inflammatory responses and enhancing regulatory T-cell activity.
• Activating the OGF-OGFr pathway regulates cell cycle control and promotes proper tissue repair.
• Promoting autophagy, the natural cellular cleanup process that declines with age.
• Providing neuroprotection by reducing neuroinflammation and supporting cognitive health.
• Improving mitochondrial function, enhancing energy production, and reducing oxidative stress.
The Peptides of “Joy and Bliss” in Longevity
Low-dose naltrexone (LDN) uniquely taps into the peptides of joy and bliss, enkephalins, and endorphins, unlocking the body’s natural ability to heal, rejuvenate, and thrive. By temporarily modulating the opioid receptors, LDN stimulates the production of these powerful peptides, enhancing mood, reducing pain, and promoting a profound sense of well-being. Simultaneously, it works on deeper cellular pathways, reducing inflammation, optimizing immune responses, and supporting neuroprotection and mitochondrial health.
Longevity Science is not about how long you are going to live. That number belongs to God. Longevity Science is about how well you live.
God Bless,
Anthony Phan MD
*Low Dose Naltrexone Society: https://ldnresearchtrust.org
*Seeds Scientific Research Performance (SSRP):https://ssrpinstitute.org
*Teaching Old Drugs New Tricks! How to Find Novel Ways to Treat Your Patients Elizabeth Yurth, MD, FAARM, FAARFM, ABAARM: Boulder Longevity Institute
SSRP Faculty Member







Beautiful Anthony, thku for a wonderful article
Thank you Dr. Phan …. Our bodies are wonderfully made . An old article from early research. So much has progressed since the late 80’s . https://journals.lww.com/acsm-msse/Abstract/1989/04000/Enkephalin_metabolism__effect_of_acute_exercise.7.aspx